Fishing for Trout in Warming Waters - Science-Based Angling Thresholds
This article was written by Jamie Madden, who recently earned her Master’s degree from Carleton University in Ottawa, Canada, co-advised by Dr. Steven Cooke and Dr. Andy Danylchuk. Her research focuses on recreational fishing and best practices for freshwater fishes.
This is a great example of how we use science to derive best practices for catch-and-release fishing.
Background
It’s that time of year when thermometer graphics show up on social media alerting anglers to be more cautious when targeting trout as water temperatures increase. Many of the graphics point to 68°F/20°C as a cutoff for when we should stop fishing for trout, but rarely are there details presented as to how that number was derived. Where is this number coming from, anyway? Does it cover all species? How about when a trout is first fought – physiologically stressed - on the end of a line?
Depending on species, fish can have a large or small range of water temperatures that they can generally tolerate. Science is often conducted to determine optimal temperatures for growth and feeding, and the outcomes can provide insights into changes in fish behavior and their reactions to warming global temperatures. One major caveat here is that many of these studies test the effects of water temperature without the additional stresses that are related to angling. So, while the preferred or optimal temperature range of a trout may be appropriate for most of its life, the higher end of that range may present challenges during and in recovery from an angling event.
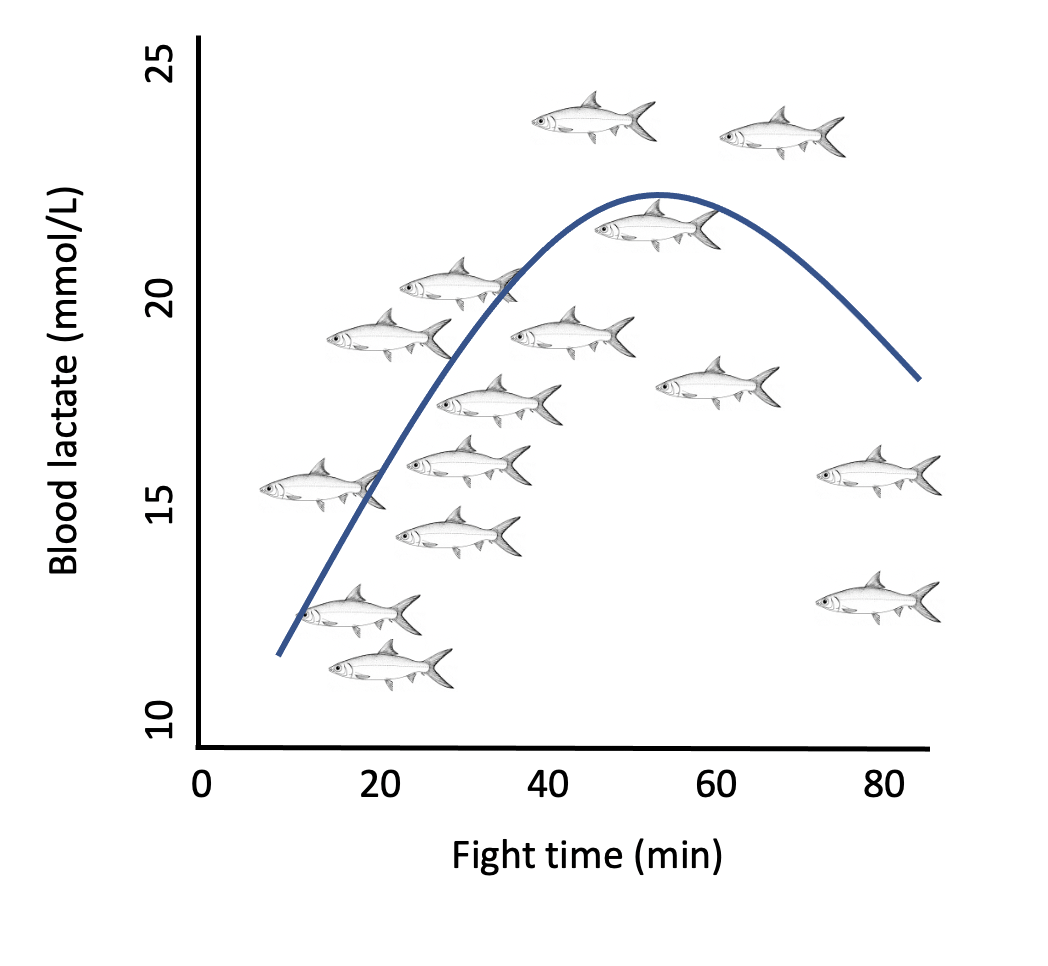
Fish undergo exhaustive exercise during angling causing their demand for oxygen to increase. In warmer temperatures, dissolved oxygen may be harder to come by, which can be one factor increasing mortality if the fish is released. In addition to exhaustion, air exposure time in warmer temperatures can result in increased lethal and sublethal (i.e. longer recovery times, reflex impairment, higher stress and lactate levels) effects compared to similar times in cool water (see this study and this study).
This brings us back to the 68°F/20°C angling cutoff that gets shared. Does it fail to take into consideration the activity requirements of a fish being angled? Where is the scientific evidence behind this number?
With that, I was worried that the information being offered to anglers may be over-generalizing at best, and inaccurate at worst. Trout anglers should and do care a lot about the wellbeing of the fish they release, and many carry a thermometer to check the water temperature. State agencies and angling organizations are also starting to promote Hoot Owl regulations to pause trout fishing when water temperatures exceed a certain temperature.
This growing level of awareness is great, however the decisions we make as anglers should be driven by scientific evidence so that we trust that our actions are indeed making a difference. It is this uncertainly that prompted my deep dive into the published scientific studies to see how much we know about how different species of trout respond to being angled at warmer water temperatures. My findings may surprise you.
What I did
Specifically, using Google Scholar I conducted a thorough review of the scientific literature to find research that combined temperature tolerance and exercise for rainbow trout, cutthroat trout, brook trout, brown trout, bull trout, and steelhead. After screening, I extracted all relevant information from each study, including species, location, continuous or constant temperature, type of study (field or lab), angled or exercised, primary variables studied, temperature range, sample size, holding method, mortality percentage, and sublethal effects.
Pooling all the data points from the studies, I produced graphs comparting mortality rates and water temperatures for each species. When there was enough data, the angling threshold was selected for when mortality began to rise exponentially, usually around 5-10%. The angling threshold indicates a tipping point for fish — it’s when water temperatures begins to be critical as evidenced by the rapid increase in mortality with every degree increase in water temperature.
The scientific papers are linked here.
What I found
There are varying levels of research done on temperature tolerance during and after angling or exhaustive exercise for the 6 species in this literature review. Notably, research for brown trout and bull trout is severely lacking. I attempted nonetheless to determine an angling threshold from comparisons with similar species, though more research is needed to confirm or adjust this range. Here are the main takeaways from the review:
Although there are slight differences among thermal tolerances of steelhead, rainbow, brook, and cutthroat trout, the angling thresholds (the temperature that indicates the beginning of an exponential rise in mortality) are lower than the 68°F/20°C commonly shared.
I found that the angling threshold is around 61°F /16°C for steelhead, rainbow, brook, and cutthroat trout. This number was determined from a combined 24 mortality studies and 9 sublethal impact studies and keeps temperature-based mortality under 5-10%.
Brown trout are known to withstand slightly warmer temperatures. With the data available (4 mortality studies and 1 sublethal effects study), I suggest an angling threshold of 66°F/ 19°C*.
Comparatively, bull trout occupy a significantly colder niche than the other species examined. Combining the mortality results from one 2020 study with sublethal data and feeding temperature ranges, I suggest an angling threshold of 54°F/12°C* for bull trout.
*This is our best guess from the limited current literature combined with known life history information of this species. More research is needed to confirm or adjust this number.
Take home message
Cynically, the temperature stopping point for fishing is a bit like a “choose your own ending” novel with the outcome being “choose your preferred mortality rate”. Instead, I’ve presented the concept of an angling threshold — a temperature at which mortality begins to increase exponentially (usually going above 5-10% just from temperature) and at which anglers need to start being exceedingly aware of every interaction they have with fish. Some anglers may also decide that this is when they want to stop fishing.
The results of my literature review suggest the angling threshold of 68°F/20°C is too high for all the species I researched.
I encourage anglers to be more conservative and start being aware of the impacts of temperature of fish much earlier, specifically at 61°F /16°C for rainbow, steelhead, cutthroat, and brook trout, 66°F/ 19°C for brown trout, and 54°F/12°C for bull trout.
While the Keep Fish Wet Principles are always relevant, specific catch-and-release best practices are ever-evolving. Undoubtedly, we need more purposeful research with the aim to determine or confirm species-specific best practices on water temperature, especially for brown trout and bull trout. There also needs to be much more research on the effects of water temperature, angling induced exercise, and other elements of the angling event, such as air exposure, since the potential impacts from angling are cumulative. For now, I encourage anglers to pay attention to the fish in their hands, be observant, be conservative when the science is behind, and be advocates for the use of current science-based best practices when targeting trout.